Our panel of in vitro end-point studies assess the antitumoral effect of your lead compounds, as well as their mechanisms of action on cell cycle, or on the immune system. Our capacity to perform multiple colour flow cytometry helps you characterising your target cells, and indications.
Our offer includes
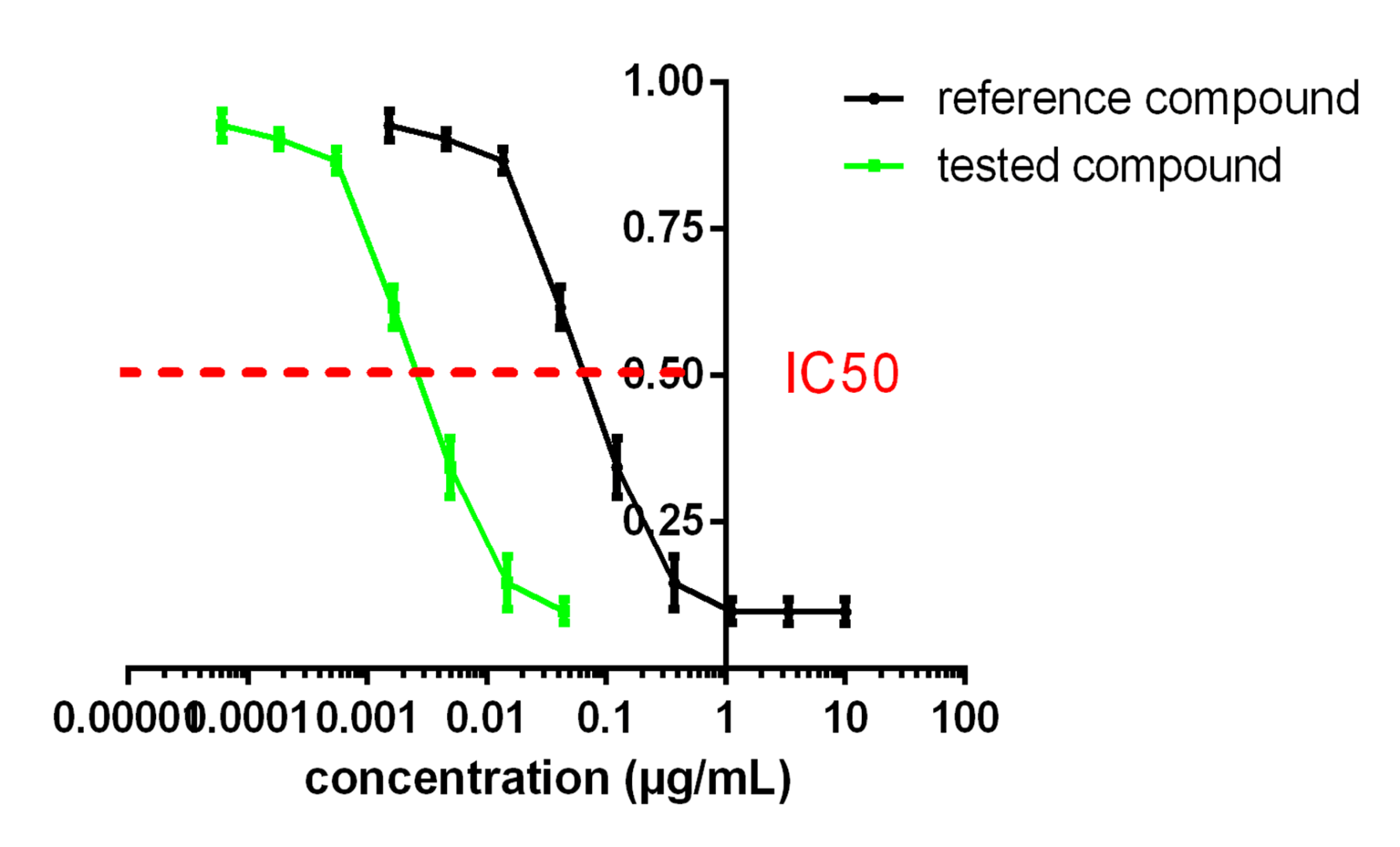
- Determination of cytotoxicity and IC50
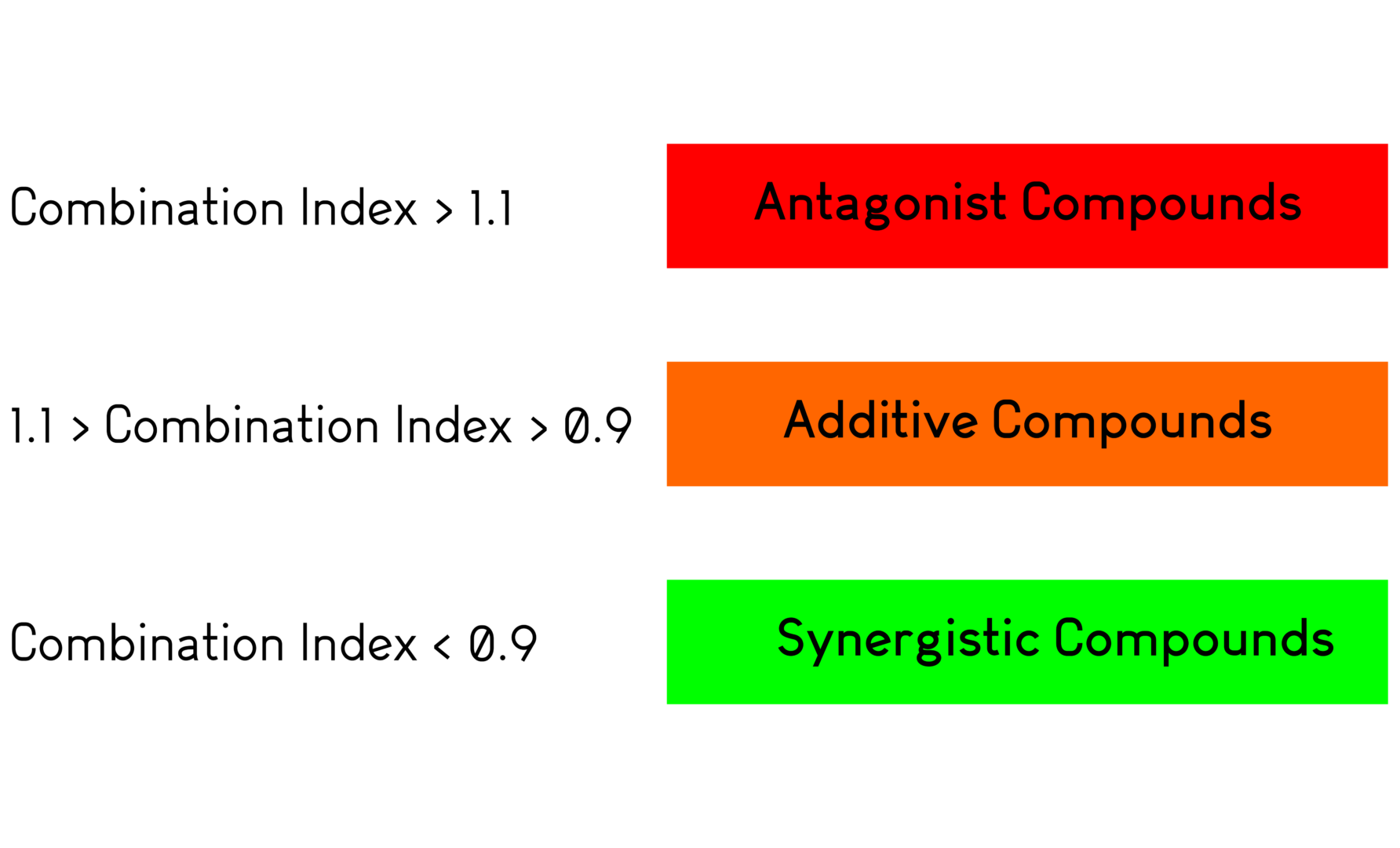
- Synergy assays between compounds or with gold standards
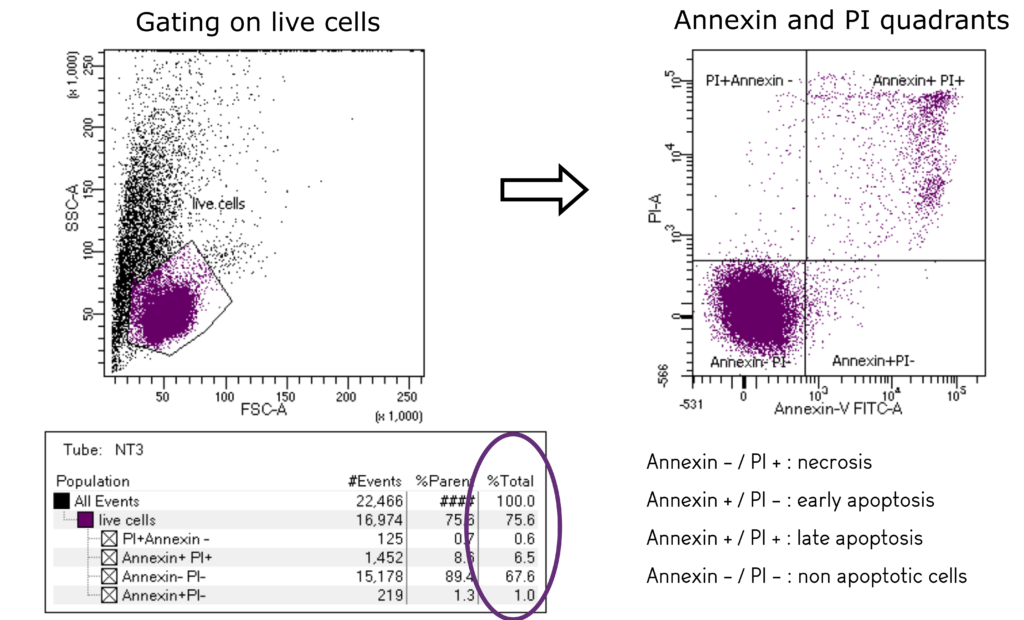
- Analysis of cell cycle mechanisms (apoptosis, stasis, proliferation, phases modifications)
- Multiple colour flow cytometry
- Immunology and immuno-oncology assays (T cells activation, CTL assay, etc)

 Antineo
Antineo Preclinical services
Preclinical services Tumour models
Tumour models Our Strengths
Our Strengths News & Events
News & Events