A major step in preclinical drug development is demonstrating the antitumor activity of a novel agent in animal models, alongside with the choice of the optimal dosage and treatment schedule that balance efficacy and safety.
Antineo in vivo preclinical services include:
We provide a complete set of in vivo preclinical services designed to support the evaluation and optimization of novel oncology therapeutics. Our services address multiple critical checkpoints, from selecting the appropriate tumor models and assessing systemic toxicity, to evaluating antitumor efficacy and exploring potential combination therapies. We also offer immunophenotyping of the tumor microenvironment, ensuring that your compound is extensively characterized regarding immunological interactions within the tumor.
Here is an overview of the key in vivo services we offer:
Recommendations on the choice of the best indication and model
Selecting the right preclinical tumor model is essential for generating relevant and predictive data. Based on our expertise, we provide guidance to help you choose the most appropriate models based on your compound’s mechanism of action and the cancer types you wish to target. Whether you are developing immunotherapies, small molecules, or biologic agents, we can help you identify the best indication and tumor model to put all the chances on your side for the success of your preclinical studies. Our selection includes both human-derived xenograft (CDX) models, syngeneic murine models, and patient-derived-xenografts (PDX) models.
Systemic and hematological toxicity of your compounds in rodents
A crucial aspect of preclinical development is evaluating the toxicity profile of preclinical compounds. Our rodent studies allow assessing the systemic toxicity of your compounds, including both acute and chronic exposure scenarios, to identify any adverse effects or organ toxicity that may arise. We also evaluate hematological toxicity, analyzing potential impacts on blood cell counts and immune function, helping you assess the overall safety of your compound before moving forward to clinical trials.
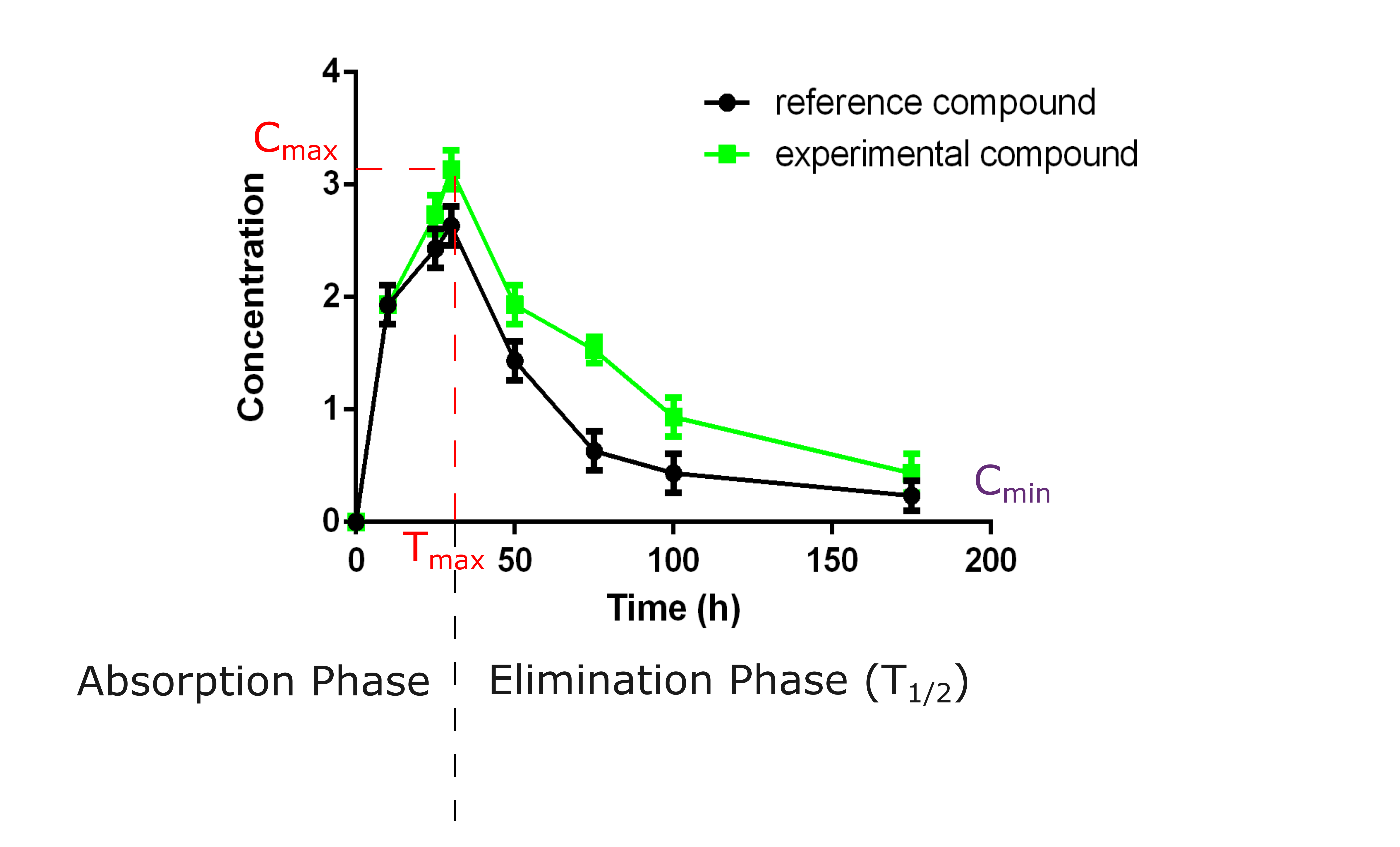
Pharmacokinetics (PK) properties in mouse and rat
The understanding of the PK properties of your compound is essential for designing effective dosing regimens in clinical settings. We conduct PK studies in mice and rats to assess how your compound is absorbed, metabolized, and eliminated from a whole organism. After an analysis by mass spectrometry, the results will help define the half-life of your compound, a critical point for optimizing the dosing schedule and ensuring therapeutic efficacy while minimizing toxicity.
Antitumor efficacy in human or murine tumor models
A key component of preclinical oncology research is assessing the antitumor efficacy of your novel therapeutic. We use a variety of human or murine tumor models to evaluate the effectiveness of your compound in inhibiting tumor growth or inducing tumor regression. Our CDX models, patient-derived xenografts, and syngeneic models are designed to replicate the tumor biology and immune landscape of specific cancer types, providing insights into how your compound will perform in humans.
Combination/comparison with Standards-of-care
To evaluate the relative efficacy of your novel compound, we can conduct combination studies or comparative studies using gold standard treatments in various cancer types. Whether comparing a novel agent with a chemotherapeutic, immune checkpoint inhibitor, or targeted therapy, these studies allow us to assess how your compound performs in combination or as an alternative to current therapeutic options, providing valuable data on synergistic effects, treatment resistance, and potential patient benefit.
A characterized catalogue of more than 150 models
More than 100 human-derived CDX models and 40 syngeneic mouse models are available in our database. These models include a variety of solid tumors, including breast, lung, colorectal, and melanoma, as well as hematologic malignancies (e.g., leukemia and lymphoma). These well-established models offer a robust platform for researching the toxicity, immunological responses, and effectiveness of drugs in various tumor types.
An original offer of unique secondary resistances models to reference therapies
As resistance to treatment is one of the most significant challenges in oncology, we offer secondary resistance models that mimic the acquired resistance seen in patients who initially respond to treatment but eventually relapse. Our unique CDX and syngeneic models of secondary resistance to reference therapies (e.g., immune checkpoint inhibitors, chemotherapy) allow to investigate the mechanisms of resistance and enable the evaluation of new therapies that may overcome this challenge.
The development of on-demand models
We also offer custom development of new models, and in particular resistant tumor models to a standard of care of interest for you or to your own therapy to evaluate specific mechanisms of resistance in immunotherapy and chemotherapy. These models are ideal for testing novel compounds that aim to reverse resistance or sensitize tumors to current therapies.
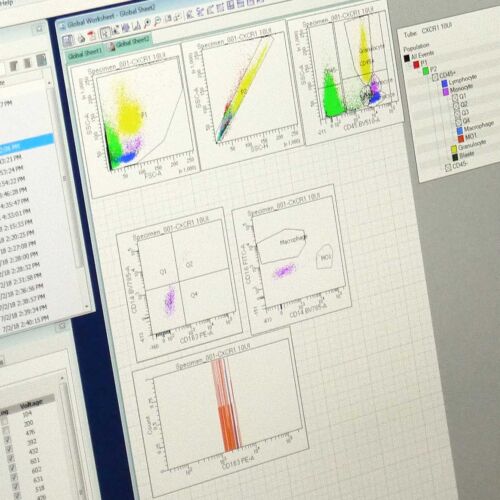
Immunophenotyping of the tumor microenvironment
Understanding the tumor microenvironment (TME) is important for developing therapies that not only target tumor cells but are also able to modulate the immune system within the tumor. We offer immunophenotyping studies of the TME, including the analysis of immune cell populations, cytokine profiles, and immune checkpoint markers in both tumor tissue and peripheral blood. These studies can help you understand how your therapy affects the immune landscape and its potential to enhance antitumor immunity.
To learn more about how Antineo can support your in vivo preclinical research, or to discuss your specific needs, contact us.

 Antineo
Antineo Preclinical services
Preclinical services Tumour models
Tumour models Our Strengths
Our Strengths News & Events
News & Events